Universal Influenza Vaccine Candidate Advances
Promising results in a recent study from Krammer et al. report their chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial
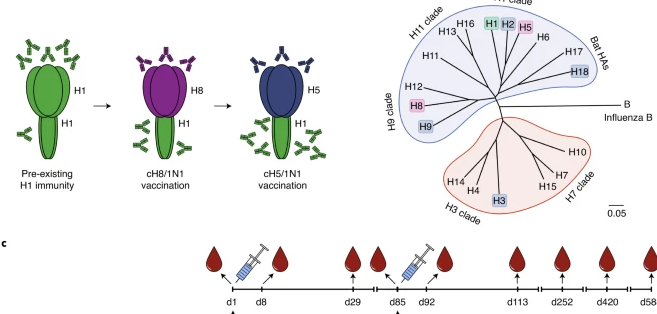
An innovative UIV strategy has cleared an important hurdle, announced December 7th, 2020 in Nature. A vaccine aimed at a stable feature of multiple influenza viruses—and therefore not subject to the mutations that necessitate annual reformulation of flu vaccines—was found to induce a strong immune response in a Phase I safety trial. The result, hailed as a significant first step on the long road to a UIV, provides a foundation for a vaccine that could protect against the full spectrum of influenza viruses.
As the humanitarian and economic toll of COVID-19 continues to mount, this breakthrough offers hope of preventing another, even more destructive pandemic. Influenza is widely recognized as a paramount pandemic threat—a matter of when, not if—and a UIV is the best way to stop it in its tracks. COVID-19 has also shown us how rapidly the vaccine R&D ecosystem can respond to such a threat, but we can do better. We can protect the world from the influenza pandemic we know is coming.
We must sustain the unprecedented commitment of scientific, technical, and financial resources brought to bear on COVID-19 and apply it to the pursuit of a UIV. In particular, we must embrace new funding models that reflect the vast advantage of investing in a UIV to prevent a pandemic, relative to influenza’s potential to trigger catastrophic economic losses. Now is the time to accelerate UIV development.
Related Resources
Episode 3: Now is the Time to Protect the World from Influenza
Astonishing progress toward successful COVID-19 vaccines has built momentum to conquer infectious disease threats. In our latest video, infectious disease experts Harvey Fineberg, Julie Gerberding, Edward Holmes, Michael Osterholm and Lynda Stuart say a full-court press for a universal influenza vaccine should be next.
